- Accueil
- Recherche
- Group leaders
- Prof. Pierre-Yves BOCHUD
- Immunogenetics of Fungal Infections
Prof. Pierre Yves Bochud
Professeur associé
Médecin chef
Service des maladies infectieuses
Université de Lausanne (UNIL)
CHUV
BH10/537
Rue du Bugnon 46
1011 Lausanne, Suisse
Immunogenetics of Fungal Infections
Aspergillus species and other mold (e.g. Mucorales) can cause life-threatening infections among patients undergoing intensive chemotherapy for the treatment of hematological cancer and/or hematopoietic stem cells (HSCT) or solid organ (SOT) transplantation. Broad-spectrum azoles are recommended to prevent invasive mold infection (IMI) in at-risk populations, but their use is limited by an unfavorable drug-interaction profile, cost and emergence of resistance. Several centers rather use a less toxic, mold-inactive, prophylaxis (preventing yeast infections), together with a pre-emptive strategy (serological and radiological surveillance) to ensure prompt treatment when an IMI is suspected. Altogether, more targeted strategies are needed to optimize the use of antifungals in this population.
It has long been unknown why some patients rapidly develop IMIs, while others, under similar immune-suppressive conditions, do not. In fact, variants in immune genes can influence the capability of certain individuals to fight fungal pathogens. We showed that single nucleotide polymorphisms (SNPs) within an innate immune gene involved in fungal detection, TLR4, were associated with susceptibility to IMI among hematopoietic cell transplant (HCT) recipients. Among other, investigators showed an association between functional SNPs in long pentraxin-3 (PTX3) and IMI in HSCT recipients. PTX3 SNPs had the advantage to be more frequent than TLR4 SNPs, making them easier to use for clinical applications. We then showed that SNPs within PTX3 were associated with susceptibility to IMI among SOT patients from STCS as well as acute leukemia in patients from the isolation Unit in CHUV. Altogether, these results make PTX3 polymorphisms robust genetic markers for IMIs and open the way to personalized management strategies.
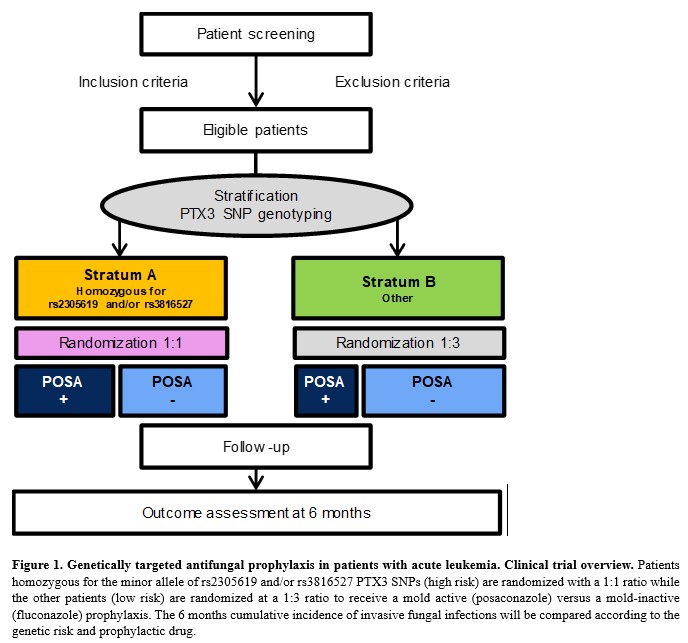
Through a SNF funding (IICT), we are currently performing “real life” PTX3 genotyping to optimize the use of antifungals in the prophylaxis of patient with acute leukemia. Genetic testing is performed in our lab for patients diagnosed with acute leukemia and prophylaxis during chemotherapy-induced neutropenia is determined according to PTX3 genotypes rs2305619 and/or rs3816527. This approach could provide a rationale for antifungal prophylaxis in leukemia patients, by limiting the use of broad-spectrum agents to high-risk patients, while avoiding their unnecessary side effects, costs and emergence of resistance in the other patients.
Selected publications
Tschopp J, Brunel AS, Spertini O, Croxatto A, Lamoth F, Bochud PY. Clin Infect Dis. 2022 Aug 25;75(2):330-333. doi: 10.1093/cid/ciab1028. PMID: 34996098. View article
Brunel AS, Wójtowicz A, Lamoth F, Spertini O, Neofytos D, Calandra T, Marchetti O, Bochud PY. Haematologica. 2018 Nov;103(11):e527-e530. doi: 10.3324/haematol.2018.195453. Epub 2018 Jun 7. PMID: 29880612. View article
Wójtowicz A, Lecompte T, Bibert S, Manuel O, Berger C, Boggian K, Cusini A, Garzoni A, Hirsch HH, Weisse M, Mueller NJ, Meylan PR, Steiger J, Kutalik Z, Pascual M, van Delden C, Bochud PY, and the Swiss Transplant Cohort Study. Clin Infect Dis. 2015:61 ;619-22. PMID: 25977268. View article
Bochud PY, Chien JW, Marr KA, Leisenring W, Upton A, Rodrigues S, Li S, Hansen JA, Zhao LP, Aderem A, Boeckh M. NEJM 2008; 359:1766-77. PMID: 18946062. View article
Team
- Aurélie Guillet (Study Nurse)
- Dre Stéphanie Bibert (PhD)
- Leyder Lozano-Calderon (Laboratory technician)
Collaborators
- Dr. Dionysios Neofytos (Geneva)
- Dr. Anna Conen (Aarau)
Open positions
- No current position available



