- Accueil
- Recherche
- Group leaders
- Dre Tatiana Katérina GALPERINE
- Risk assessment of Dientamoeba fragilis transmission through fecal microbiota transplantation and its consequences in recipients (FMT-fragilis)
Dre Tatiana Katérina Galperine
Cheffe de clinique
Privat-docent, MER
Service des maladies infectieuses
Université de Lausanne (UNIL)
CHUV
BH09/788
Rue du Bugnon 46
1011 Lausanne, Suisse
Risk assessment of Dientamoeba fragilis transmission through fecal microbiota transplantation and its consequences in recipients (FMT-fragilis)
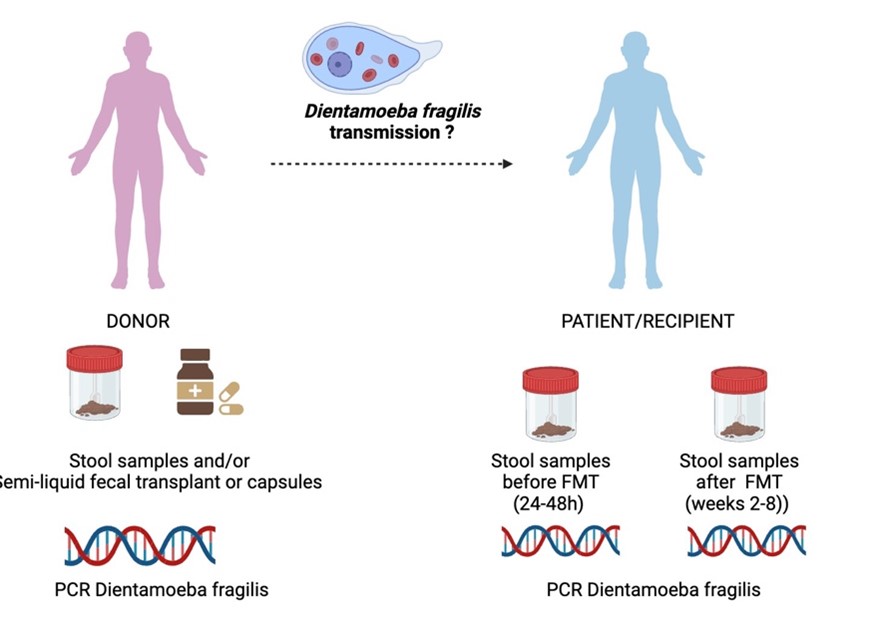
Current guidelines recommend a systematic screening of Dientamoeba fragilis organism in intestinal microbiota donors. The risk of Dientamoeba fragilis transmission through fecal microbiota transplants and the consequences on recipients in the event of transmission are not established. The presence is considered as an exclusion criterion despite the lack of evidence for its pathogenicity.
This study will be conducted on healthy volunteers selected as donors for fecal microbiota transplantation, as well as on the transplants and recipients treated with FMT. The presence of Dientamoeba fragilis will be investigated through – real-time PCR in frozen (-80°C) fecal samples from both donors and recipients, as well as on the transplants (semi-liquid or capsules). A standardized clinical follow-up of patients is conducted as part of routine care to monitor all post-transplantation adverse effects, with follow-ups at day 15 and week 8 (figure).
Main objective:
- Evaluate the frequency of detecting D. fragilis in donors, transplants after freezing, and recipients.
Secondary objectives:
- Assess the effectiveness of FMT with donors positive for D. fragilis.
- Evaluate the clinical consequences of a potential transmission of D. fragilis.
- Assess the composition and function of the intestinal microbiota in positive donors compared to donors negative for D. fragilis.
Selected publications
Team
- Swiss Tropical and Public Health institute (Swiss TPH)
- Collaboration with Pr N.Kapel; Dr A.Moreno (APHP, Paris)
Open positions
- No current position available



