- Accueil
- Research
- Group leaders
- Prof. Oriol MANUEL
- Vaccination in immunocompromised patients
Prof. Oriol Manuel
Professeur associé
Médecin adjoint
Service des maladies infectieuses
Université de Lausanne (UNIL)
CHUV
BH10/549
Rue du Bugnon 46
1011 Lausanne, Suisse
Vaccination in immunocompromised patients
Respiratory viral infections are associated with a more severe clinical presentation and higher risk for complications in SOT recipients. Influenza vaccine is the key preventive strategy against influenza, although its efficacy is reduced in SOT recipients. Our research has focused on two main aspects: 1) the assessment of the epidemiology and burden of disease of respiratory viral infections in SOT recipients 2) the improvement of vaccine immunogenicity by investigating novel vaccination approaches. We have assessed the epidemiology and outcomes of respiratory viral infections in a large cohort of SOT recipients [1]. We showed that respiratory viral infections (in particular influenza and rhinovirus) were common after transplant and associated with significant morbidity. These results suggested that the implementation of improved preventive strategies against respiratory viral infections remains an important need in transplant medicine.
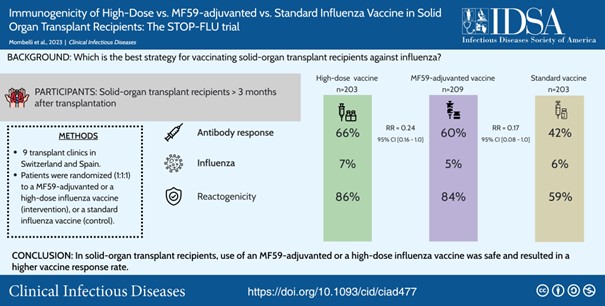
We have conducted two pilot randomized clinical trials on the use of new vaccination strategies in the transplant population. We first tested a double dose vs. standard dose strategy of the influenza vaccine in SOT recipients [2]. We also evaluated the efficacy of adding a topical adjuvant (imiquimod) to the intradermal and intramuscular vaccine in immunocompromised patients [3]. The results of these studies allowed us to plan a phase IIb/III multicentre randomized clinical trial on the efficacy, immunogenicity and safety of three commercial influenza vaccines in SOT recipients (STOP-FLU trial). This trial has been funded by the SNF (Investigator Initiated Clinical Trial Program - IICT) and enrolled 620 patients. We found that high-dose and MF59-adjuvanted vaccines elicited increased immunogenicity as compared to the standard influenza vaccine [4].
Selected publications
Mombelli M, Lang BM, Neofytos D, Aubert JD, Benden C, Berger C, Boggian K, Egli A, Soccal PM, Kaiser L, Hirzel C, Pascual M, Koller M, Mueller NJ, van Delden C, Hirsch HH, Manuel O, Swiss Transplant Cohort Study. Am J Transplant 2021; 21: 1789-1800. DOI.View article
Mombelli M, Rettby N, Perreau M, Pascual M, Pantaleo G, Manuel O. Vaccine. 2018; 36: 6163-6169. DOI. PMID: 30181045. View article
Mombelli M, Hoschler K, Cavassini M, Pascual M, Manuel O. J Infect. 2021; 83: 354-360. DOI. PMID: 34298035. View article
Mombelli M, Neofytos D, Huynh-Do U, Sánchez-Céspedes J, Stampf S, Golshayan D, Dahdal S, Stirnimann G, Schnyder A, Garzoni C, Venzin RM, Magenta L, Schönenberger M, Walti L, Hirzel C, Munting A, Dickenmann M, Koller M, Aubert JD, Steiger J, Pascual M, Mueller TF, Schuurmans M, Berger C, Binet I, Villard J, Mueller NJ, Egli A, Cordero E, van Delden C, Manuel O; Swiss Transplant Cohort Study. Clin Infect Dis. 2024 Aug 16. DOI.. PMID: 37584344. View article
Team
- Matteo Mombelli, MD, former senior investigator
- Aline Munting, MD
- Valérie Sormani, RN
Open positions
- No current position available



