- Accueil
- Recherche
- Translational research unit
- T cell biology and engineering
T cell biology and engineering
We study human CD8 T-cell responses to self-(tumor) and foreign (viral) antigens with the aim to advance our knowledge of T cell-mediated protection from human disease and to improve T cell-based therapies against cancer.
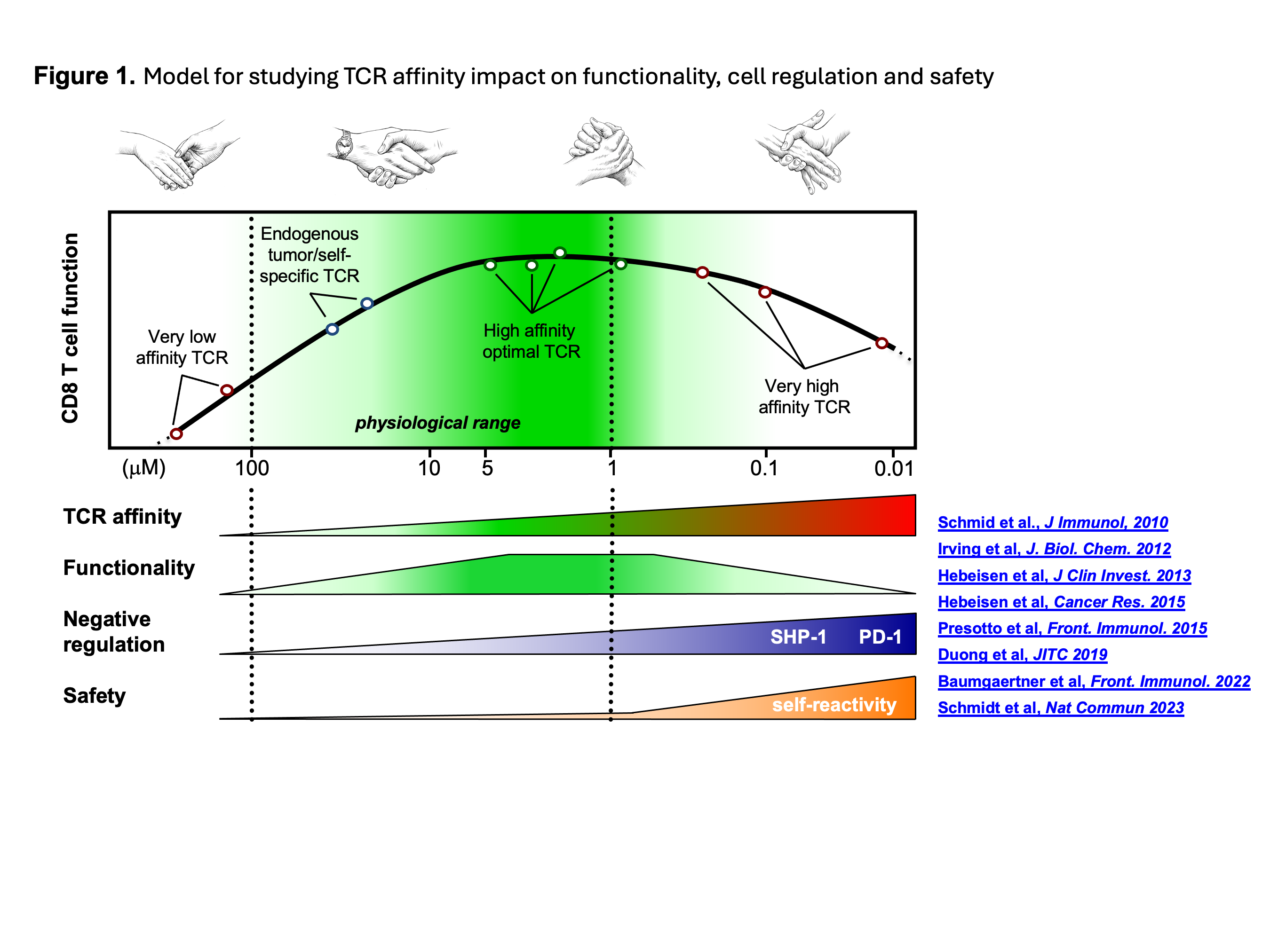
One focus lies in the optimization of anti-tumor T cells for future adoptive cell transfer to treat cancer patients. For this purpose, my team has generated a unique model of genetically engineered T-lymphocytes, by equipping them with TCRs of increased affinity against NY-ESO-1, a tumor antigen expressed in different types of cancer (Fig. 1). We were able to demonstrate that the peak function of these modified T cells is "calibrated" by regulatory mechanisms linked to TCR affinity. Moreover, sustained chronic interactions between affinity-increased TCR and self-MHC/self-proteome can directly adjust the functional potential of engineered T cells.
Altogether, our research underlines the importance of identifying safe anti-tumor TCRs capable of generating optimal function without toxicity for effective clinical application.